Your Cart is Empty
In October 2018, Purifas® conducted a clinical trial on the difference in bacterial transfer from the client to the therapy bed with and without the use of the FaceShield™.
As part of the study, we followed a stringent three step cleaning protocol to ensure the bed was sterile before we commenced treatment to guarantee accurate data collection. A swab of the bed was taken to ensure the bed was sterile.
The first aspect of the study, physiotherapy treatment without the FaceShield™, replicates the current lack of protection around the face hole, with a swab taken after five patients to determine bacterial transfer (Figure 1).
This process was repeated with the FaceShield™ to assess the reduction in bacterial transfer as a result of using the FaceShield™ (Figure 2).
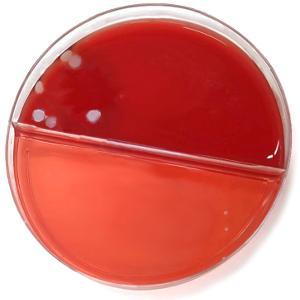
Images of the agar plates comparing the levels of bacterial growth are below. It is evident from the images that there is a significant reduction in bacterial transfer with the use of the FaceShield™.

|
 |
|
Figure 1: No FaceShield™ used |
Figure 2: FaceShield™ used |
As the FaceShield™ filters bacteria in both directions, if the bed was infected with pathogens prior to commencing therapy, our FaceShield would also protect the consumer as it reduces their exposure to these microbes.
Ensuring top-notch hygiene in therapy and allied health settings is vital for client safety. Shared equipment can easily lead to cross-contamination without strict hygiene protocols. Towels, frequently used in these environments, can harbour bacteria even after washing, posing risks to both clients and therapists. This guide will help you understand these risks, avoid common mistakes and implement the best hygiene practices, all while being environmentally conscious.